Https www fda gov downloads aboutfda reportsmanualsforms forms ucm048364 pdf South Algonquin

DEPARTMENT OF HEALTH AND HUMAN SERVICES Silver Spring IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission
Overview of FDAAA and Other Trial Registration Policies
To Sign or Not to Sign FDA Form 1572? Applied Clinical. DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400, Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research.
U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research
Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission
IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research
01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission
1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research
To Sign or Not to Sign FDA Form 1572? Applied Clinical

Introduction to Postmarketing Drug Safety Surveillance. Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research, Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research.
DEPARTMENT OF HEALTH AND HUMAN SERVICES Silver Spring. U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA, IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission.
Approval Letter BLA 761040 Food and Drug Administration

Silver Spring MD 20993 Food and Drug Administration. IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission 1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II.

Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400
01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA
ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop 01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of
IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research
U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400 Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research
01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400
ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400
Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400
Approval Letter BLA 761040 Food and Drug Administration
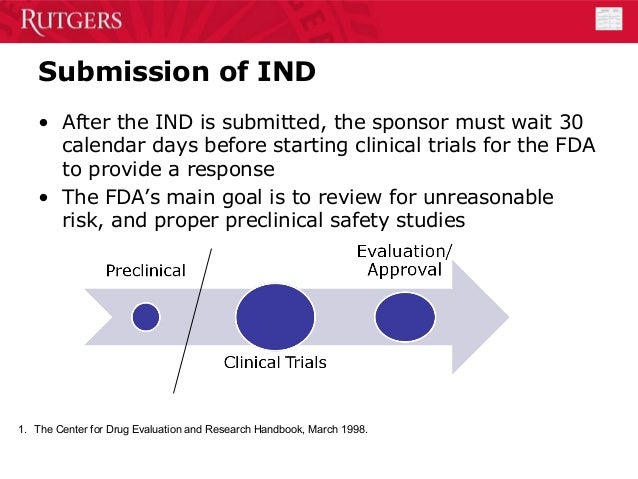
Form FDA 3779 accessdata.fda.gov. Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research, IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission.
DEPARTMENT OF HEALTH AND HUMAN SERVICES Silver Spring
To Sign or Not to Sign FDA Form 1572? Applied Clinical. Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research, ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop.
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission
ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400 1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II
U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400
1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA
U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research
Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA
01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of 01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of
Approval Letter BLA 761040 Food and Drug Administration
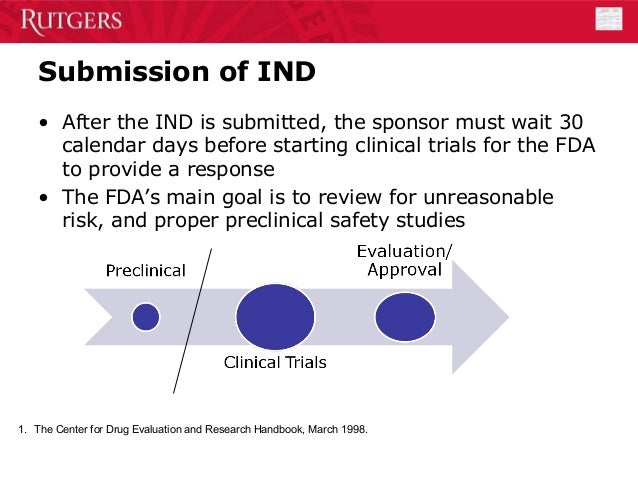
Introduction to Postmarketing Drug Safety Surveillance. DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400, Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research.
Approval Letter BLA 761040 Food and Drug Administration. DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 209176 . NDA APPROVAL . Mitsubishi Tanabe Pharma Development America, Inc. Attention: Douglas N. Dobak US Agent for Mitsubishi Tanabe Pharma Corporation Vice President, Head of Regulatory Affairs and Quality Assurance 525 Washington Blvd, Suite 400, U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA.
Introduction to Postmarketing Drug Safety Surveillance

INITIAL INVESTIGATIONAL NEW DRUG APPLICATION. IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission.

U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA 1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II
Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission
Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA
Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research Food and Drug Administration Center for Drug Evaluation and Research Division of Compliance Risk Management and Surveillance 5901-B Ammendale Road Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration Center for Drug Evaluation and Research
U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II Food and Drug Administration . Center for Drug Evaluation and Research . Division of Compliance Risk Management and Surveillance . 5901-B Ammendale Road . Beltsville, MD 20705-1266 . Biological product deviations, sent by courier or overnight mail, should be addressed to: Food and Drug Administration . Center for Drug Evaluation and Research
ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop 01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of
1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II ClinicalTrials.gov Results Database Train-the-Trainer Workshop September 2015 1 Overview of FDAAA and Other Trial Registration Policies Results Database Train-the-Trainer Workshop
1 Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER Kelly Cao, Pharm.D. Safety Evaluator Team Leader. Division of Pharmacovigilance II U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA

IND Title (if title is being used) Serial 000. Name of Sponsor-Investigator, MD. X Professor, Department. University of California, Los Angeles (Note to User: This template is only intended for вЂsimple’ INDs where commercially marketed drugs are being evaluated by sponsor-investigators)Date of Submission 01/07/2018В В· There has previously been no alternative non-U.S. regulatorВґs statement available; therefore, the instructions on the FDA 1572 form itself and the recommendation from the FDA Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs: Frequently Asked Questions: (Form 1572) of May 2010 were, in general, followed by sponsors of


